Self-Sorting and Self-Assembly: Chiral Metallosupramolecular Squares and Multiply Threaded Pseudorotaxanes
Christoph A. Schalley
Professor of Organic Chemistry Institute for Chemistry and Biochemistry
Free
The self-assembly of supramolecular architecture requires suitably programmed building blocks. The first part of the talk focuses on the highly specific self-assembly of chiral metallo-supramolecular squares. This includes dynamically chiral ligands which self-sort upon square assembly in a heterochiral way (Figure, left).
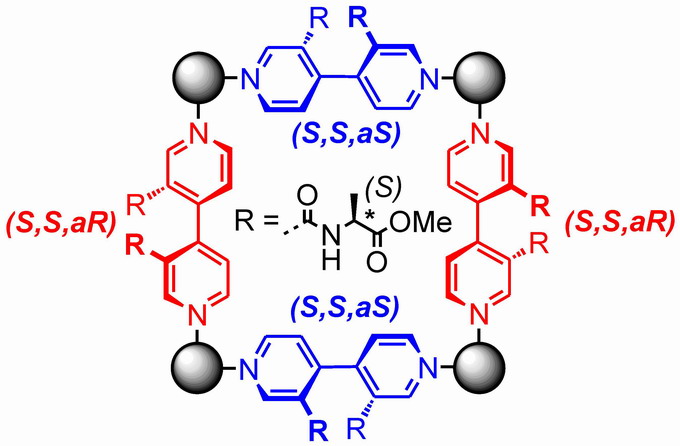 | 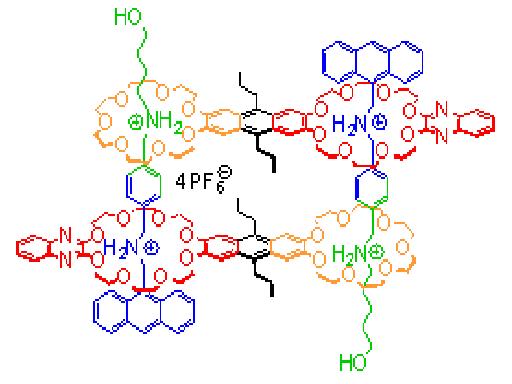 |
The integration of different, orthogonal binding sites in one building block enhances our ability to program. Multiply threaded pseudorotaxanes (Figure, right) can be assembled with full spacial control based on two binding motifs: The smaller 21-crown-7 cannot slip over a the central phenyl group of the divalent axle. Consequently, only the larger 24-crown-8 ether ends up in the neighborhood of the anthracene. In the example shown, this determines both axles and both crown ether dimers to be antiparallel in the thermodynamically most favorable assembly. A mass spectrometric micro-reactor technique can monitor error correction steps during the formation of the assemblies.
( Sep. 20, 2010)

近期发表论文:
Nature Chem. 2010, 2, 533-538
J. Am. Chem. Soc. 2010, 132, 2309-2320
J. Am. Chem. Soc. 2010, 132, 484-494
Angew. Chem. Int. Ed. 2009, 48, 7246-7250
Nature Chem. 2009, 1, 573-577