New approach to synthesizing inorganic monoliths
Prof. TANG Ruikang and Prof. LIU Zhaoming from the Zhejiang University Department of Chemistry discovered that amorphous calcium carbonate particles can be fused into integrated monoliths via regulation of structurally bound water and external pressure. The strategy of using the material’s own structural idiosyncrasies to promote mass transportation overcomes the flaw of conventional sintering and provides a novel approach to manufacturing inorganic bulk materials.
Their research findings were published in the June 25 issue of Science.
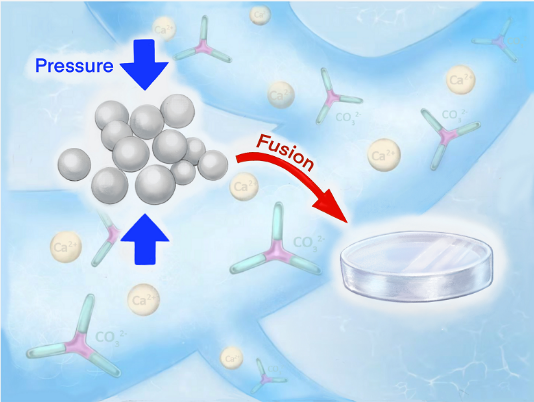
Calcium carbonate is one of the common substances on the earth and it is also a principal component of animal bones or shells. Meanwhile, as an inorganic compound, it is also extensively used in industry.
When calcium carbonate is manufactured by existing artificial methods, only micron-sized white powder can be obtained in most cases. However, bulk materials are needed in practice in many cases.
“Elastic and malleable organic materials are relatively susceptible to deformation. Inorganic compounds like calcium carbonate are hard and brittle, thus making it hard to manufacture them into monoliths,” TANG Ruikang said, “In a myriad of repairs involving inorganic materials, such as heritage conservation and dental restoration, organic materials are more widely used. They may look identical to original organic compoundssubstrates in appearance. However, they don’t have “the same rootthe same composition” as original ones, so they are more vulnerable to crack and damage due to their poor intrinsic incompatibility.”
Using inorganic materials in repairs remains to be the essential way out. The core problem to be solved lies in the preparation of inorganic block materials.
Inorganic materials are traditionally produced in powder forms and then consolidated by pressing and sintering. However, mass transportation among particles is often insufficient, and complete particle-particle fusion cannot be achieved within bulk materials, thereby affecting the mechanic performance of sintered inorganic bulks. Moreover, many temperature-sensitive biominerals and biomaterials cannot be manufactured by high-temperature sintering methods.
Scientists have endeavored to find an ideal solution in nature. An increasingly large body of research revealed that biological organisms can use amorphous particles as precursors to produce inorganic skeletons that have continuous structures with flexible morphology. Inspired by this intriguing biological phenomenon, TANG Ruikang et al. proposed that monolithic inorganic materials be manufactured by fusion of their amorphous precursors at normal temperature.
In the previous study by TANG Ruikang et al., they developed “inorganic ionic polymerization”. This method can be used to manufacture centimeter-sized calcium carbonate crystal materials quickly in the laboratory. Furthermore, this preparation method is marked by salient moldabilityplasticity. This study was published in the journal Nature in October 2019.
It is in this study that MU Zhao discovered a mesmerizing phenomenon that the boundaries of inorganic calcium carbonate particles would gradually vanish and fuse completely into each other during the preparation process.
Researchers found that water played a vital role in inducing crystallization of amorphous particles. Although scientists had noticed this phenomenon before, there was no in-depth research into the relationship among the function, mobility and structural stability of structural water. Further research by MU Zhao and KONG Kangren indicated that if there are an appropriate number of water molecules, a dynamic water channel will be formed inside calcium carbonate, thus facilitating the transportation of internal materials and contributing to the ultimate fusion of amorphous particles. “Without sufficient water content, channels cannot form. However, too much water forms a much largernew water cluster that induces the crystallization of amorphous calcium carbonate particles.”
Water regulation can be achieved via ordinary heating. When one calcium carbonate molecule corresponds to 0.2-1.1 water molecules and P ranges from 0.6 to 3.0 GPa, fusion of amorphous calcium carbonate particles can be achieved. Accordingly, researchers constructed calcium carbonate bulk materials with a continuous structure.
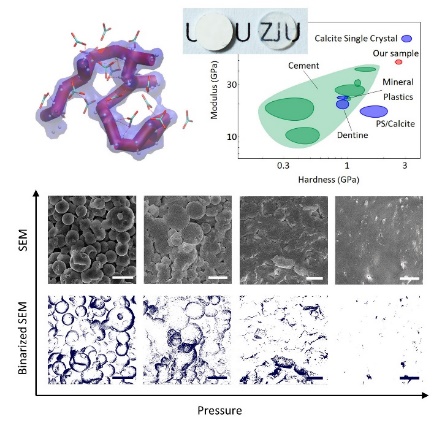
Calcium carbonate monoliths produced in this way are featured by structural continuity. They exhibit optimal transparency and remarkable mechanic performance with a hardness (H) of 2.739 GPa and a Young’s modulus (E) of 49.672 GPa. These values are superior to those of common cement materials and close to those of single-crystal calcite. What’s more, this method can be employed at normal temperature and in a quick manner. “If required pressure can be lowered in the future, it will be more feasible,” KONG Kangran Kangren said.
“This work helps us better understand and mimic biological mineralization. Take, for example, the genesis of transparent teeth in chinook salmons, one of the top predators in the deep sea. Both the deep-sea high-pressure environment and amorphous minerals suggest the conditions for the formation of such teeth with a continuous structure.
Meanwhile, the discovery of dynamic water channels in the study gave rise to a new possible way of material existence: liquidoid. Popular notion has it that a solid is a solid and a liquid is a liquid, with a clear boundary between the two. But in the future, there may be an intermediate state between solids and liquids.

Prof. TANG Ruikang, Prof. LIU Zhaoming and their team
"Further experiments revealed that fusion applies to a wide range of inorganic ionic compounds. Besides water molecules, other ions can also be added as additives, which will affect the fluidity and fusion of calcium carbonate. This demonstrates a potential way to improve the fluidity of solid materials and presents new knowledge regarding the fusion of solid materials. Solid inorganic materials are expected to have liquid-like properties at room temperature." This study reflects the assets of amorphous phases in materials processing and offers a new alternative to producing artificial bulk materials which has potential applications in biology, medicine, and materials.